Introduction:
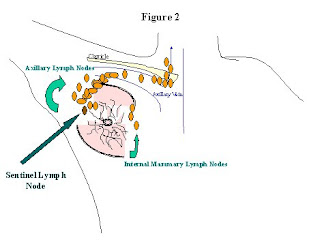
When discussing the surgical treatment of your breast cancer with you, your surgeon will discuss whether or not your breast cancer is invasive. Breast cancers can be confined within the lining of the endothelial cells along the breast duct (in-situ cancers); or it can start to spread beyond the breast duct (invasive cancers). This is important because the blood vessels and lymph vessels that potentially spread the cancer beyond the breast run along this area (See Figure 1). If the cancer has spread beyond the lining of the breast duct, and is picked up by the blood vessels or lymph vessels, then it can potentially spread elsewhere in the body, or “metastasize.” Lymph vessels are small channels that drain all the tissues of the body. Lymph vessels drain excess fluid back into your circulation. As lymph fluid drains back into your circulation, it goes through lymph nodes. Lymph nodes are collections of lymph tissue that have a high concentration of white blood cells, the cells in your body that fight infection and cancer. The lymph vessels of the breast drain into the lymph nodes in your axilla (underneath your arm), and sometimes into the lymph nodes along your sternum, (or breastbone), and above your clavicle (collarbone) (See Figure 2).

Axillary Lymph Node Dissection:
Traditionally, if your breast cancer is invasive, an axillary lymph node dissection is recommended by your surgeon in order to see if the cancer has spread to the lymph nodes underneath the arm. During an axillary lymph node dissection, the surgeon makes an incision underneath your arm, and removes the bulk of the lymph node tissue that drains from the breast. The lymph node tissue is then sent to the laboratory, and a pathologist looks at the lymph nodes under a microscope and determines if any of them contain cancer. On average, approximately 10 to 15 lymph nodes are removed with this operation. An axillary lymph node dissection usually requires an overnight stay in the hospital. Since the remaining tissues underneath the arm tend to “leak” some lymph fluid when the lymph nodes are removed, a drain is left in place for the first 2-3 weeks after the operation until the area heals. The drain is a flexible plastic tube that exits the skin, and is connected to a plastic collection bulb. When the drainage diminishes to a certain amount, the drain is removed in the clinic. After you go home you are given physical therapy exercises to maintain strength and flexibility in your shoulder while this area heals. Approximately 5-10% of the patients who undergo an axillary lymph node dissection experience chronic problems related to the dissection such as arm swelling (lymphedema), or pain or discomfort in the area of the dissection. Almost all women will have some residual numbness under the inside of the arm.
Sentinel Lymph Node Biopsy:
A sentinel lymph node biopsy is a new technique. This was developed as a test to determine if breast cancer has spread to the lymph ducts or lymph nodes in the axilla without having to do a traditional axillary lymph node dissection. Experience has shown us that the lymph ducts of the breast usually drain to one lymph node first, before draining through the rest of the lymph nodes underneath the arm. That first lymph node is called the sentinel lymph node. That is the lymph node that helps sound the warning that the cancer has spread. Lymph node mapping helps identify that lymph node, and a sentinel lymph node biopsy removes only that lymph node. The sentinel lymph node is identified in one of two ways, either by a weak radioactive dye (technetium-labeled sulfur colloid) that can be measured by a hand held probe, or by a blue dye (isosulfan blue) that stains the lymph tissue a bright blue so it can be seen. Most breast cancer surgeons use a combination of both dyes. This procedure is new. The “best” way to administer the dye, which dye to use, and the benefits and risks of the procedure in various situations is still being studied. A traditional axillary lymph node dissection is the “tried and true” method, and is still considered the “gold standard”.
Advantages:
The advantages to the sentinel lymph node procedure are many. There is no need to stay overnight in the hospital. There is no need for a drain, or physical therapy exercises. Your recuperation from the procedure is faster. You are typically doing your regular activities within a few days, and the incision is well healed within a few weeks. A sentinel lymph node biopsy can lead to a more accurate assessment of whether the cancer has spread to the lymph nodes. In a traditional axillary dissection, the pathologist receives at least 10 lymph nodes or more; there is no way of telling which one is the sentinel lymph node. So the pathologist makes one cut in each lymph node and looks for cancer. When the pathologist receives only one, or a few, lymph nodes from a sentinel lymph node procedure, he or she can make many cuts through that lymph node to look for cancer. A negative sentinel lymph node(s) indicates a >95% chance that the remaining lymph nodes in the axilla are also cancer free. Therefore, there is no need to undergo a full axillary lymph node dissection, or to risk the long term complications and side effects from an axillary dissection.
What to Expect:
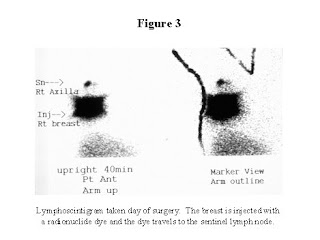
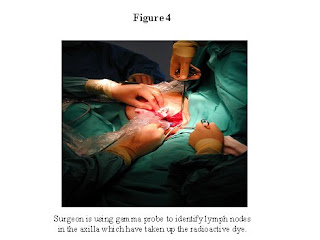
If you decide to undergo the procedure, the morning of your operation, you will go see a nuclear medicine specialist who is a physician specifically trained in injecting the radioactive dye used for the procedure. The injections are done into the area of the breast where the tumor is, and/or around the nipple areolar complex of the breast. You will then return to the nuclear medicine department a few hours later, and pictures will be taken which show the pathways the dye takes as it leaves the breast. (See Figure 3) This will help guide your surgeon in identifying the sentinel lymph node. Then you will proceed to the operating room. At the beginning of the operation, your surgeon will inject the blue dye. The surgeon then makes an incision underneath your arm in the area of the axillary lymph tissue. A hand-held sterile probe measures areas that have the radioactive dye. (See Figure 4) The lymph nodes that have taken up the radioactive dye, or are stained with the blue dye, are removed. Usually one to three nodes are removed. These nodes are sent to the pathologist, who then looks at them under a microscope to see if the sentinel node contains cancer. Your incision is closed, and there is no need for a drain. There is no need for physical therapy exercises. Unless you are having another operation done which requires that you stay overnight, you can go home from the hospital that day. The sentinel lymph node biopsy can be done in combination with a lumpectomy, or a mastectomy. The procedure is successful in >90% of those patients whom we think are good candidates for the procedure. If the procedure is unsuccessful in identifying the sentinel node, a full axillary dissection is done.
Who Shouldn’t Undergo the Procedure:
Unfortunately, the sentinel lymph node biopsy procedure can’t be performed on everyone with an invasive breast cancer. People who have had radiation therapy or surgery in their breast or axilla should not undergo the technique, as changes in the breast and axilla from the radiation therapy or surgery may make the results inaccurate. People who have enlarged lymph nodes underneath their arm, or people who we know already have breast cancer metastatic to their axillary lymph nodes should undergo a traditional axillary lymph node dissection. People who already have had a mastectomy can’t undergo the procedure because there is no accurate way to inject the dye to identify the lymph node. People with large tumors (greater then 5cm) have a higher incidence of lymph node spread of their cancer, and may be better served by a traditional lymph node dissection. They should discuss this with their surgeon. People, in whom it will be difficult to accurately inject the dye, would likely be better served by a full axillary lymph node dissection. This includes those people in whom we are unable to find the primary breast tumor (an “occult” malignancy), and people in whom the tumor is dispersed through more then one area of the breast (a multifocal tumor).
If the Sentinel Lymph Node is Positive:
If the pathology results show that the breast cancer has spread to the sentinel lymph node, then typically you will need to return to the operating room to undergo a complete axillary lymph node dissection. This is done to remove the remaining lymph nodes, which may contain cancer. Since the majority of patients with breast cancer involving lymph nodes receive chemotherapy, the new question for breast cancer specialists has been whether or not it matters if there is cancer in any of the other lymph nodes within the axilla. The answer is that we don’t know yet. There is currently a large national clinical trial called Z0011, which is evaluating exactly that question. In this trial some patients are chosen (randomized) to receive a full axillary lymph node dissection if their sentinel lymph node is positive. Some patients are chosen to not undergo any further lymph node dissection, and they are carefully watched. All of these patients will receive chemotherapy. The two groups of patients will be compared to see if there is a benefit to doing the full axillary dissection if the sentinel lymph node is positive. Or, alternatively, they will be compared to see if there is a detriment to not doing a full axillary dissection if the sentinel lymph node is positive. This is still experimental, and should only be done under controlled circumstances after being enrolled in this trial by a participating breast cancer surgeon. Doing a full axillary lymph node dissection if the sentinel lymph node is positive is still considered “the standard of care” with which all patients should be treated. To do otherwise, risks under treating your breast cancer.
Who Should Do the Procedure:
One of the factors that influences the results obtained with the procedure, is the qualification of the breast surgeon doing the procedure. Initial studies have shown that most surgeons need to do 20-30 sentinel lymph node biopsy procedures before obtaining accurate results using the technique. Surgeons can perform these cases during an accredited residency or fellowship at an institution that does a large number of these cases a year. Alternatively, surgeons attend a conference to learn the technique, then acquire these 20-30 cases as part of a training protocol. During the training period, the surgeon will perform the sentinel lymph node biopsy, and then complete a full axillary lymph node dissection at the same operation. After obtaining the pathology results, the surgeon can then determine if the sentinel lymph node was correctly identified. In addition, the surgeon can determine that the cancer was found in the sentinel node, and not in the lymph nodes that would otherwise have been left behind (false negative rate). After a surgeon has done 20-30 cases in which the sentinel lymph node is identified in >90% of the cases, and the false negative rate is less then 5%, then the surgeon “goes off protocol”, and does sentinel lymph node biopsies without a full axillary dissection. Until the surgeon has completed a large number of cases, and determined her/his accuracy for doing the technique, any cases done “off protocol” may inaccurately determine if there has been spread of cancer to the axillary lymph nodes. It is therefore important, to ask your surgeon if they have done a large number of cases during an accredited residency or fellowship, or if they have completed their learning protocol for the technique. No matter how the training for doing the procedure was acquired, there is some evidence to show that the surgeons who continue to perform this procedure on a regular basis, will have more accurate results from the technique
Summary:
Invasive breast cancer can spread through the lymph ducts and blood vessels to other areas of the body. The sentinel lymph node is the first lymph node that the lymph ducts drain into. Whether or not the cancer has spread to the sentinel lymph node indicates whether the cancer has started to spread beyond the breast. A new technique called sentinel lymph node biopsy identifies this lymph node, and allows only this lymph node to be removed. Removing only the sentinel lymph node can allow breast cancer patients to avoid many of the complications and side effects associated with a traditional axillary lymph node dissection. Patients with invasive breast cancer should discuss sentinel lymph node biopsy with their surgeon.